 GEMMOLOGICAL LABORATORY FACT SHEET
GEMMOLOGICAL LABORATORY FACT SHEET
Chemical composition of Opal:
Si0 2nH 20 – Hydrated Silica.
• Water content in Precious Opal 3-10%.
• Water content in Potch or Common Opal 2-20%
• Impurities are Oxides of:
Aluminium (~2.5%), Calcium (~1%), Sodium (~0.4%), Iron (~0.3%), Titanium and Magnesium (~0.1%) and in much smaller traces Zirconia, Manganese, Copper, Nickel and Cobalt.
Chemical composition of Boulder Opal:
SiO 2 at 28%, Fe 2O 3 + Al 2O 3 at 68%, H 20 at 4% approximately.
Hardness on Mohs Scale 1-10:
Opal is 5.5 to 6.5 on the Mohs Scale (Note* A relative not an absolute scale)
| Scratch Hardness (Mohs) |
Mineral used for resistance Comparison |
Simple Hardness tester |
Cutting |
| 1 | Talc | Can be scratched with fingernail | 0.03 |
| 2 | Gypsum | Can be scratched with fingernail | 1.25 |
| 3 | Pearl, Bone | Can be scratched with copper coin | 4.5 |
| 4 | Fluorite, Marble | Easily scratched with knife | 5.0 |
| 5 | Glass, Obsidian | Can be scratched with knife | 6.5 |
| 6 | Opal, Turquoise, Garnet | Can be scratched with steel file | 37 |
| 7 | Quartz,Tourmaline | Scratches window glass | 120 |
| 8 | Topaz, Aquamarine | Can be scratched with corundum | 175 |
| 9 | Sapphire, Ruby | Can be scratched with diamond | 1,000 |
| 10 | Diamond | Can be scratched with diamond | 140,000 |
Specific Gravity (SG):
• White or Black Opal 2.10-2.30
• Boulder Opal 2.60-2.80
Note* SG indicates the relation between the measured gemstone and an equal amount of water, its numerical value is between 1 and 8, values under 2 are considered light, those from 2 to 4 normal and over 4 heavy.

Cleavage:
• None in White or Black Opal.
• Distinct in Boulder Opal which may split along an Opal vein to produce two faces of Opal, due to the angle of the Opal vein’s microstructure growth in the cavity.
Referred to as ‘Splits’ or a ‘Split Pair’. When the colour bars or veins are true, that is fairly flat and straight; the splitting operation performed by an expert lapidary will result in the Opal remaining entirely adhered to both sides of the host rock.
Fracture: Conchoidal (glass-like)
Lustre: Vitreous (glass-like)
Streak:
• White or colourless for White or Black Opal
• White or colourless on the top-side and brown on the bottom-side for Boulder Opal
Diaphaneity: Transparent, Translucent or Opaque
Optic Character: Opal is a singly refractive gem. Light which enters an Opal remains as non-polarized beams, and travels through all directions of the stone at the same speed.
Refractive Index (RI): 1.37 – 1.47; Opal has no birefringence, each one has a single RI figure in this range.
Pleochroism: None.
Note* Pleochroism is caused by the double refraction of light and should not be confused with the play of colour which characterises Opal.
Crystal System: Opal is an Amorphous mineral, it does not occur in a particular crystallographic form. It is a replacement mineral which occurs in veins, nodules and fossils. Potch or common Opal does not have an orderly atomic structure, only precious Opal has a regular internal structure.
Atomic Structure: Electron microscope Studies (40,000x magnification) have revealed Opal is composed of tiny spheres of transparent hard silica, 0.01 to 0.0005 mm in diameter, sitting in a bath of hydrous silica.
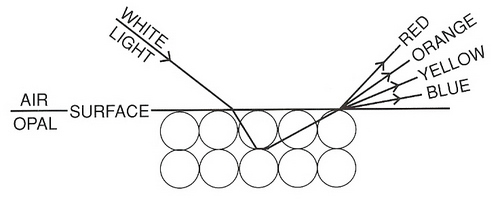
Light passes through the spheres in a straight line but when it hits the spaces between the spheres, containing silica in solution; it is bent and deflected at different angles. The overall effect of the silica arrangement is to produce a diffraction grating which breaks up white light into its constituent parts producing the visual phenomenon known as ‘play of colour’. According to the sizes of the spheres, varying colours of the spectrum are diffracted. Red colour is attributed to larger spheres ~4000 Å in diameter, while green opal spheres are ~2500 Å. Note*(Angstrom units; 1 Å = 10 -7mm) The size of the spheres diminishes through the spectrum; RED_ORANGE_YELLOW_GREEN_BLUE_INDIGO_VIOLET. Violet is attributed to the smallest spheres.
Electron Micrographs:
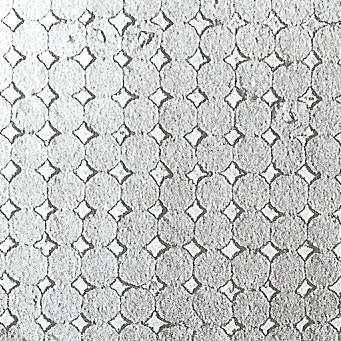 |
 |
| Precious Opal | Common Opal |
 |
 |
In precious opal the spheres are uniformly sized and arranged in an orderly 3 dimensional grid, whereas in common opal the spheres vary in size and shape, discovered by Australian CSIRO scientists Darragh and Sanders in 1965.
Field Observation: Opal seams generally show a layered structure similar to the horizontal bedding in its sedimentary host rock. Layers may differ from adjacent layers in body colour, transparency, or colour patterns.
Fluorescence:
Black and Boulder Opal do not fluoresce.
Crystal and Light Opal, mostly from South Australia, fluoresce a cloudy white under U.V. light.
Phosphorescence:
Fluorescence in South Australian Opal (origin: Andamooka, Coober Pedy, Mintabie) is normally followed by a rather prolonged phosphorescent afterglow.
This phosphorescent afterglow is hardly perceptible in Gilson Light Opal.
Opalescence: The milky-blue or pearly appearance of common opal, opalescent glass and moonstone is caused by internal reflection of short wave, mainly blue light. This optical effect is due to the scattering of light by particles of matter in its path, such as is caused by a ray of light illuminating dust particles in the air of a room. Opalescence should not be confused with play of colour.
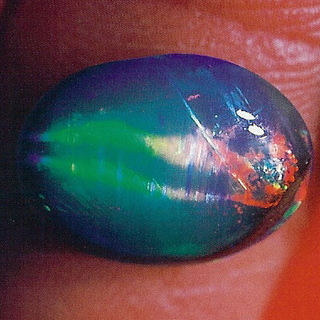
Chatoyancy: The Cat’s eye effect is caused by the reflection of light from multiple parallel needles or fibres which are inclusions in the stone. This phenomenon is most effective when the stone is cut en cabochon in such a way that the base is parallel to the fibres. A chatoyant Opal may otherwise be referred to as having a rolling-flash pattern, when the gem is rotated the cat’s eye glides over the face. Because Opal is predominantly cut en cabochon Cat’s eye Opals are not the rarest occurence, though good examples with play of colour are hard to come by.


Asterism: Created through reflection of light by thin fibrous or needle-like inclusions that lie in various directions.
There are 4-rayed, 6-rayed and 12-rayed stars in Sapphires. However asterism is an extremely rare find in Opal, very few examples of Star Opals have been recorded, including 3-rayed, 4-rayed and 6-rayed stars.
Sometimes rare patterns may be termed ‘Star’ or ‘Windmill’ pattern, these are not necessarily the same phenomena, they are usually more static with broader grains (rays) and a central point which does not move upon rotation of the stone.
Detection of Modified & Man-Made Opals
Detection of Enhanced Opals
Treated matrix: Include Andamooka Matrix and Sandstone Opal from Queensland, referred to by the miners as Fairystone.
Matrix never transmits light and may be detected by their porosity, lower hardness and lower density (SG 1.98-2.05). Under magnification tiny black spots can be seen in the surface colour of these stones and poorly treated examples may show uneven patches of body tone. The back and side colour is nearly always the same as on the face of the stone (as per synthetic opal) and these stones are often cut with a flat back and bevelled setting edge.
A porous surface may be observed under loupe on all or parts of the stone.
Detection of Composites
Composites: Doublets & Triplets
![]() Generally detected by inspection of the join around the girdle of a stone.
Generally detected by inspection of the join around the girdle of a stone.
Composites have different (generally lower) specific gravity values to their natural counterparts.
Triplets are detectable when viewed from the side by the transparency of the quartz cap and planes of seperation and coloured cement may be visible. When mounted doublets will be harder to identify and may need to be inspected under magnification (by microscope or loupe) locate bubbles in the glue.
If the stone has a boulder backing, look for ironstone inclusions travelling from the face into the body of the stone which indicate it is natural. If the stone fluoresces it is a boulder doublet. A pin heated with a naked flame can be pushed into the join line it should penetrate easily if the backing is actually powdered ironstone in an epoxy resin.
Detection of Opal Simulants
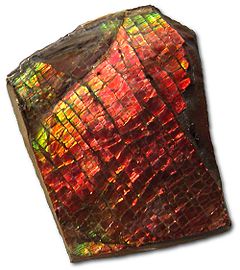
| Natural: Some multicoloured stones may appear like Opal to the untrained eye. Often these natural stimulants are presented as composites.
Labradorite Feldspar – exhibits definite cleavage and banded twinning. Ammolite – This fossilised invertebrate shell of Ammonite reminiscent of Black Opal detectable RI 1.52-1.67 and SG 2.78 Paua Shell – Much lower SG |
 |
Imitation: Opal can be confused with Glass and some plastic imitations.
Glass (aka. paste) is simply imbedded with iridescent foil or as a doublet using opalescent (milky) glass. These are easily detected on sight or with the use of a loupe.
Plastic Simulants can be similar to Gilson in appearance, yet contain substances not found in natural Opal and are much lower in density, hardness and SG. These simulants are often highly porous and have a waxy texture.
Detection of Synthetic Opal

Synthetic: ‘Gilson’ Black and White Opals have all of the gemmological properties of natural Opal and are also heat resistant. There are several points of detection for this man made product:
Freedom of inclusions like potch or sand, and a stone which looks too perfect.
Gilson White Opal does not fluoresce under UV light unlike most natural white Opal. After Longwave UV is turned off the synthetic material phosphoresces for a much shorter time than natural Opals.
Very regular patterns and play of colour, with colours always arranged in uniform layers on vertical columns.
Under magnification a mosaic pattern showing crenellated margins can be seen wihin each patch of colour, this is also described as resembling a ‘snakeskin’ or ‘fishscale’ effect.
| Sources & Image Credits:
AUSTRALIAN PRECIOUS OPAL, Andrew Cody, 1991; Diagram: Refraction Grating of Light Emil Weis Opals Collection; Photo of Opal Cats-eye GEMSTONES OF THE WORLD, Walter Schumann, 1976; diagram: Moh’s Hardness scale HANDBOOK OF GEMSTONE IDENTIFICATION, Richard T. Liddicoat, Jr., 1990 OPAL IDENTIFICATION AND VALUE, Paul B. Downing PhD., 2001. (Photo of Star Opal) THE Opaline COLLECTION; Photo of ‘Spellbound’ Boulder Opal Splits Opal Module, GAA course notes, Anthony G. Smallwood, 1998. Photo of Star Opal in tweezers, courtesy Justin Thomas. |